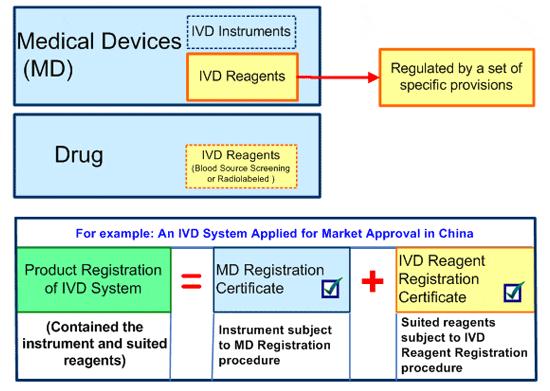
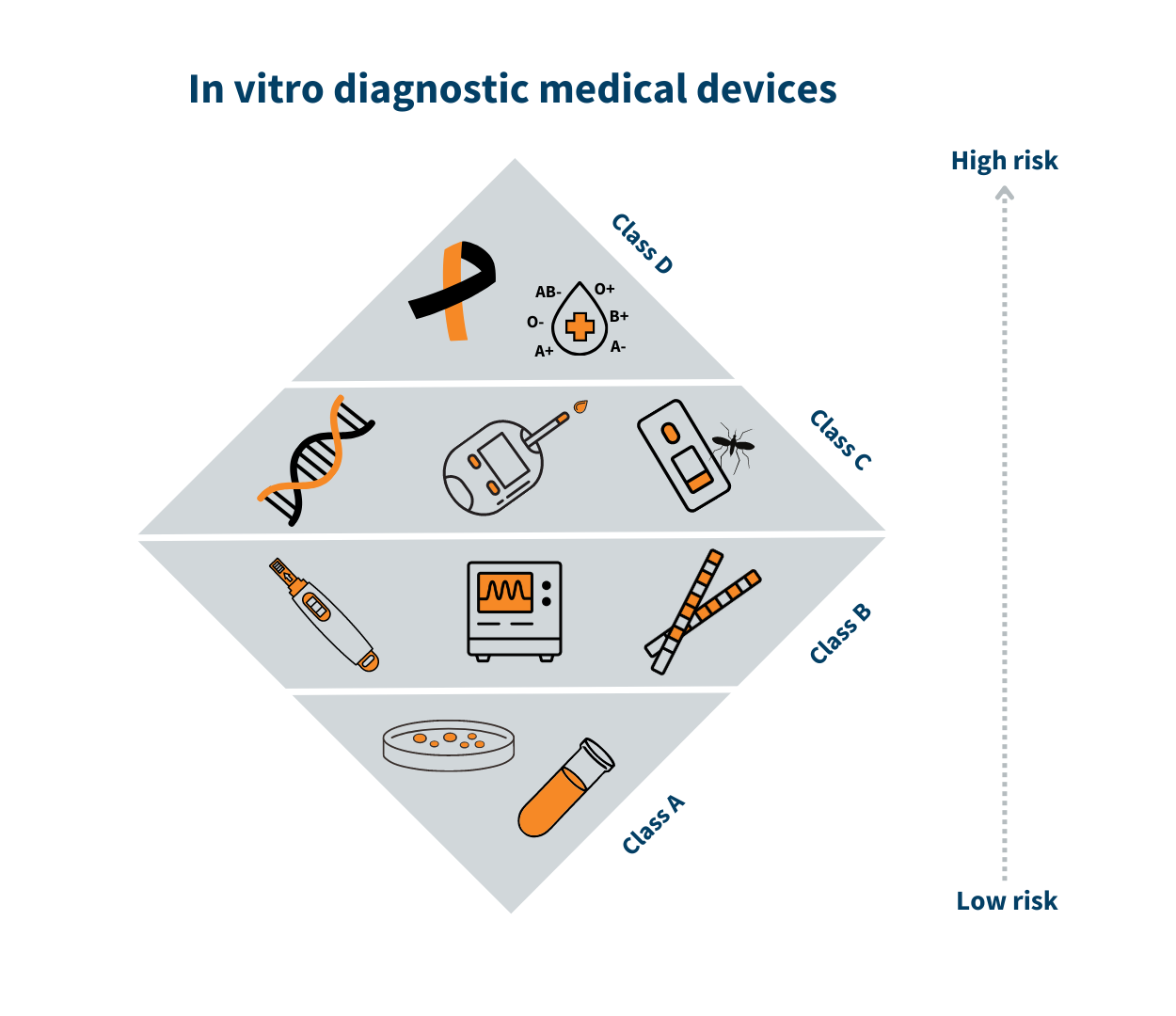
CDSCO Recently Classified 80 In-Vitro Diagnostic Medical Devices For Regulation And Patient Safety - CliniExperts -CliniExperts

Regulatory Approval and Classification of In Vitro Diagnostics India,2017: Updated Regulation for IVD Registration -