Developing Meaningful Assays: Balancing Biological Significance and QC-Compatibility - Oxford Global

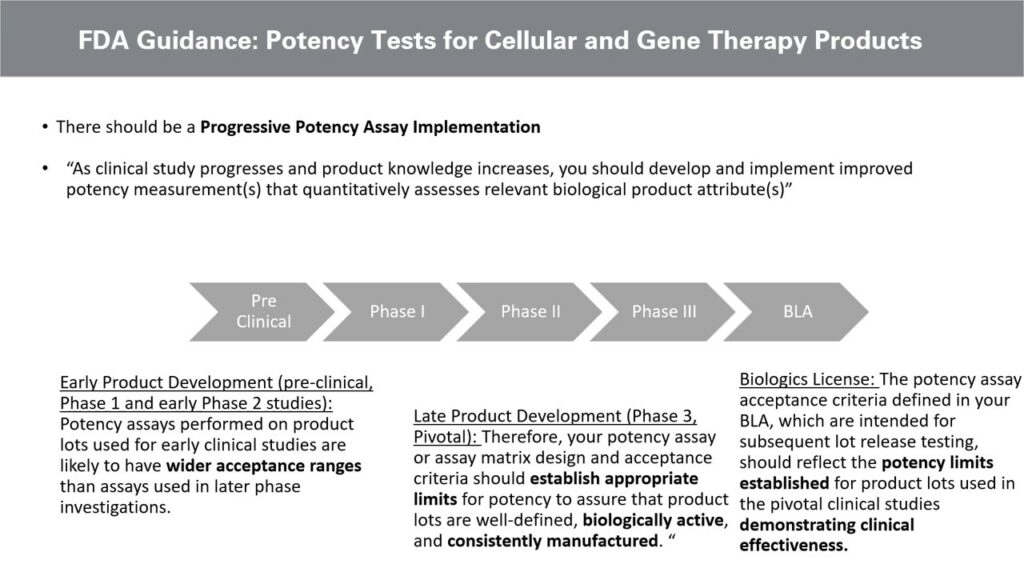
Addressing potency-assay related development delays for cell and gene therapies - Alliance for Regenerative Medicine

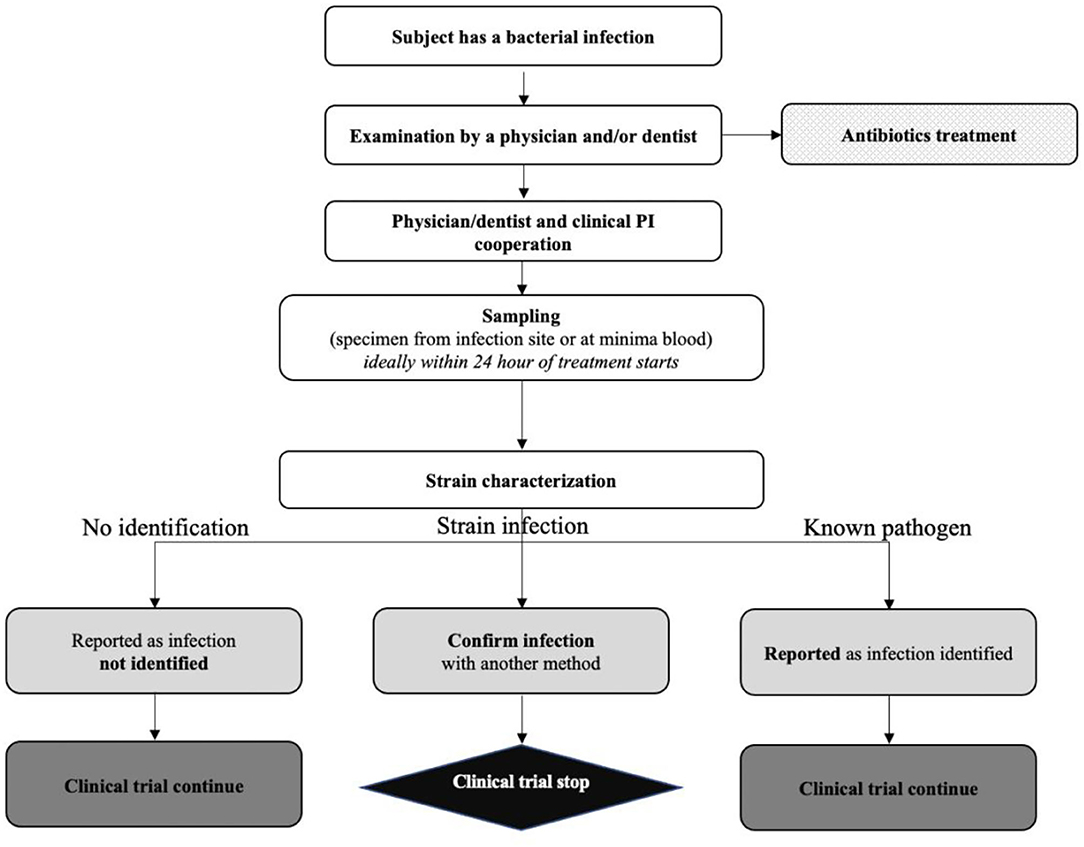
Potency assay development for cellular therapy products: an ISCT∗ review of the requirements and experiences in the industry - Cytotherapy
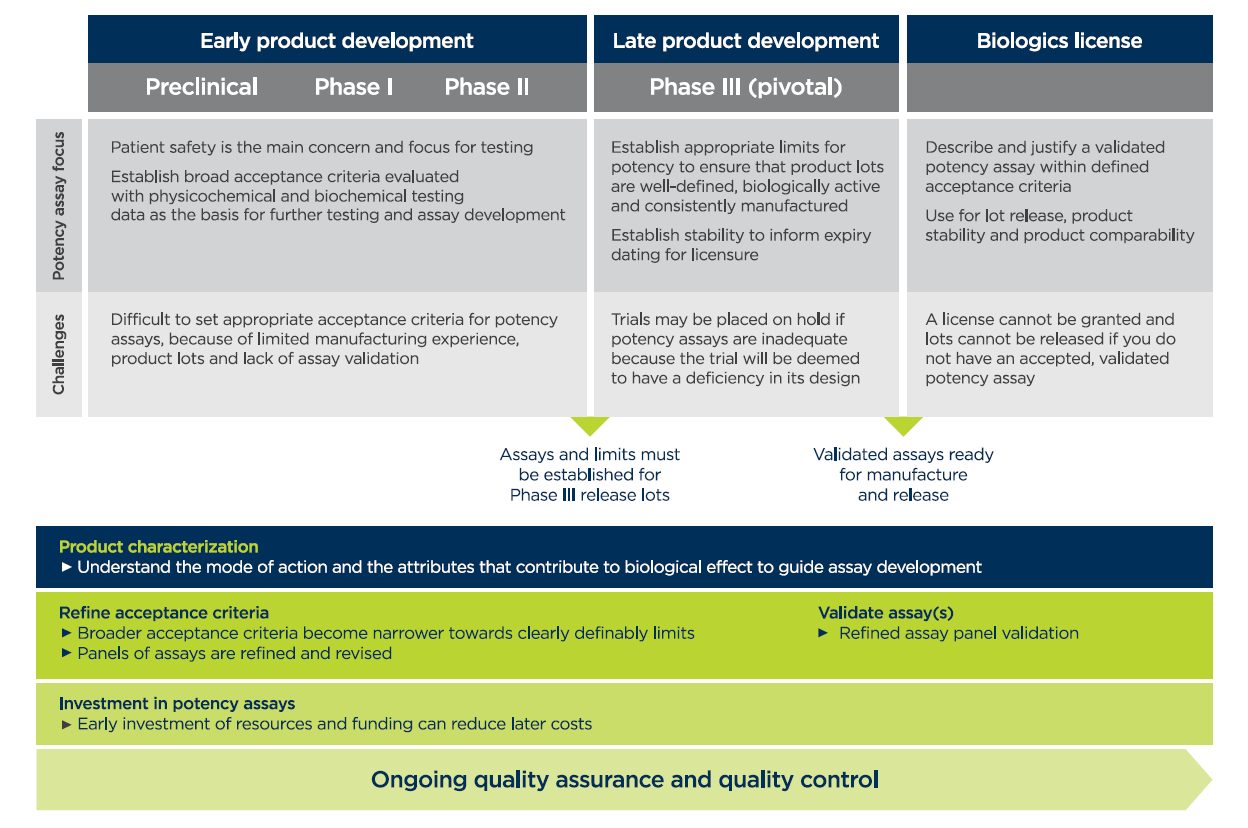
Potency assays for ATMPs: overcoming challenges on the path to commercialization - Insights From Our Labs to Yours

Potency assay development for cellular therapy products: an ISCT∗ review of the requirements and experiences in the industry - Cytotherapy

Critical considerations for the development of potency tests for therapeutic applications of mesenchymal stromal cell-derived small extracellular vesicles - Cytotherapy
.png.aspx)
Public Meeting with FDA Oct. 19 Will Focus on Potency Assays for CGTs | ASGCT - American Society of Gene & Cell Therapy

Development of an In Vitro Biopotency Assay for an AAV8 Hemophilia B Gene Therapy Vector Suitable for Clinical Product Release - ScienceDirect

Frontiers | A Regulatory Risk-Based Approach to ATMP/CGT Development: Integrating Scientific Challenges With Current Regulatory Expectations

PPT - Potency Measurements for Cellular and Gene Therapy Products PowerPoint Presentation - ID:247170

An ex vivo potency assay to assess active drug levels of a GLP-1 agonistic peptide during preclinical safety studies | Bioanalysis

Adam Feuerstein on Twitter: "Unsure if this is new information, but $PFE has not enrolled US patients into its Duchenne gene therapy ph3 study due to unresolved FDA issues w/ potency assay.
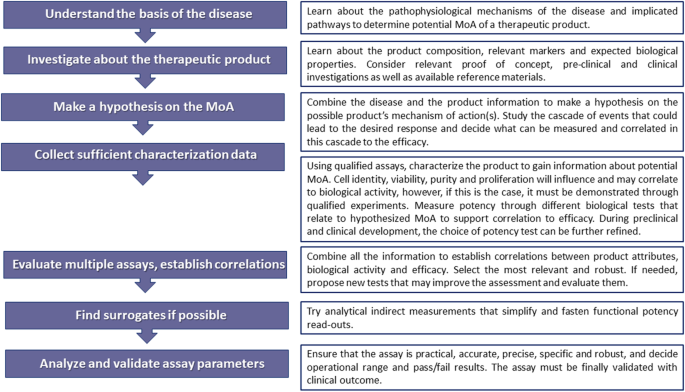
The Essential Need for a Validated Potency Assay for Cell-Based Therapies in Cardiac Regenerative and Reparative Medicine. A Practical Approach to Test Development | SpringerLink