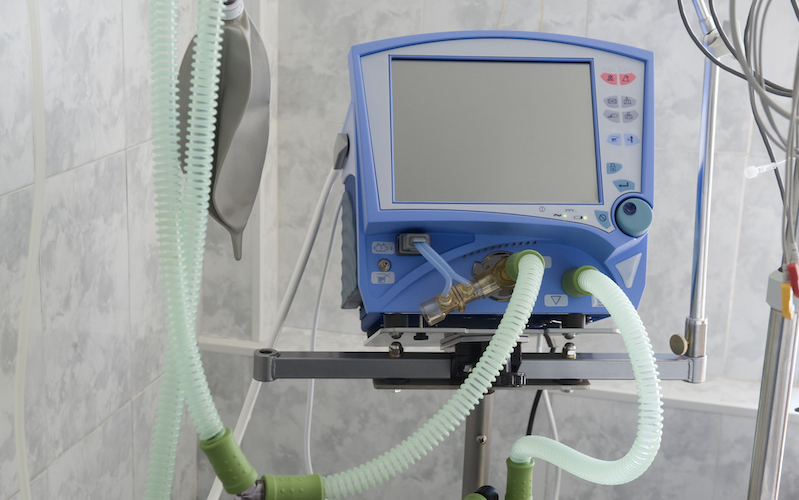
Corvent gets $4.5M to get FDA nod for shelf-stable, inexpensive, single-use ventilator | 2020-05-13 | BioWorld

FDA Eases Device Modification Rules to Expand Availability of Ventilators During COVID-19 Pandemic | Health Law Advisor
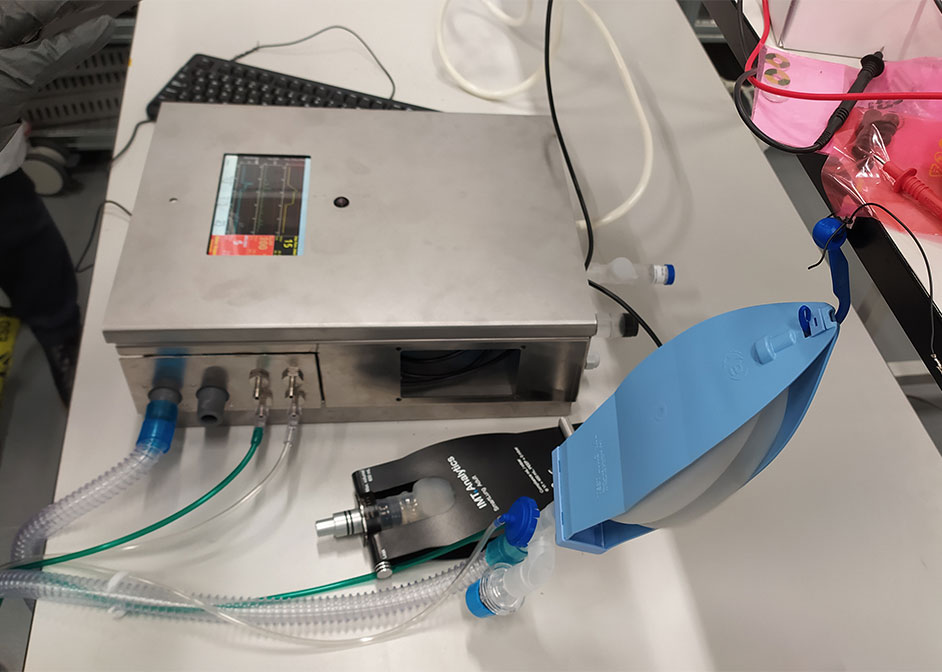
International Group of Physicists and Engineers Design FDA-Approved, Open-Source Ventilator - University of Houston
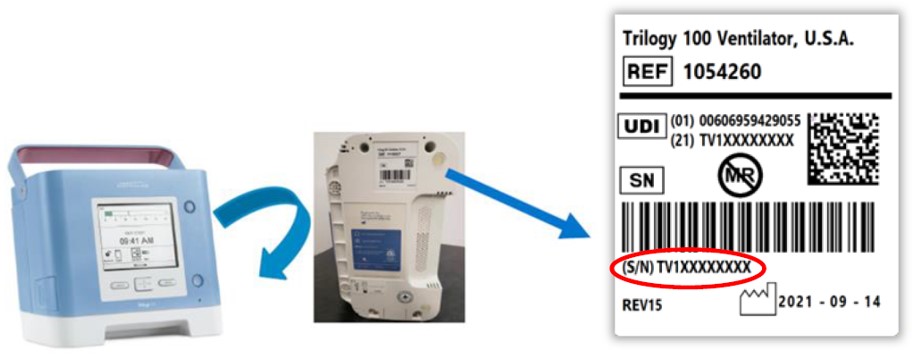
Certain Reworked Philips Respironics Trilogy 100/200 Ventilators Recalled Due to Potential for Silicone Foam Adhesion Failure and Residual PE‐PUR Foam Debris: FDA Safety Communication | FDA

Sophisticated Modeling Boosts Split Ventilators to Emergency FDA Review | Duke Pratt School of Engineering

FDA authorizes first-of-its-kind, low-cost ventilator developed by University of Minnesota | University of Minnesota